General approach to a spiro indole-3,1′-naphthalene tetracyclic system: stereoselective pseudo four-component reaction of isatins and cyclic ketones with two molecules of malononitrile†
Abstract
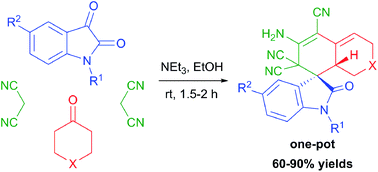
A new type of catalytic stereoselective cascade pseudo four-component reaction was discovered. The simple and facile pseudo four-component reaction of isatins, cyclic ketones with two molecules of malononitrile catalyzed by triethylamine at ambient temperature stereoselectively results in the formation of tetracyclic spirooxindoles in 60–90% yields. Thus, a new simple and efficient ‘one-pot’ method to synthesize substituted spirooxindoles was found directly from reasonable starting compounds. Unique stereoselectivety was achieved on two or three centers in this pseudo four-component reaction.

 Please wait while we load your content...
Please wait while we load your content...