One-pot protocol to synthesize N-(β-nitro)amides by tandem Henry/Ritter reaction†
Abstract
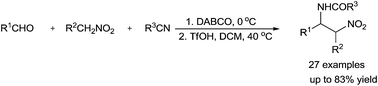
A novel, efficient and atom economical one pot protocol for the synthesis of N-(β-nitro)amides has been described by combining the Henry reaction with the Ritter reaction. The designed products could be obtained from easily available aldehydes, nitroalkanes and nitriles with 60–83% overall yields under mild reaction conditions. In addition, the product can easily be transformed into diamine or protective amine derivatives.

 Please wait while we load your content...
Please wait while we load your content...