Synthesis and characterization of micro/nano-structured BaHPO4/Ba3(PO4)2/Ba5(PO4)3OH phases and their luminescence
Abstract
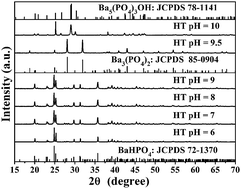
Microspheres covered with microcuboids/nanorods and nanoparticles of BaHPO4/Ba3(PO4)2/Ba5(PO4)3OH phases have been successfully synthesized by a facile hydrothermal (HT) method using the citric acid as a surfactant at different pH values. X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and fluorescence spectrometry were used to characterize the samples. It was found that the pH value was a crucial factor for the phase formation and shape determination of the final products, which were discussed in detail. Attractively, the as-prepared BaHPO4/Ba3(PO4)2/Ba5(PO4)3OH samples emitted an intense blue light in a broad band from 380 to 625 nm, for which the mechanism was complex ions luminescence originating from the transition of 3T1 → 1A1 in PO43−. Meanwhile, an obvious red shift for the emission band was observed between nano- and bulk-Ba3(PO4)2 synthesized by HT and conventional solid-state (CSS) reactions, respectively, which was due to the effect of the product being nanosized. The same effect was also revealed by the fact that the decay time of the latter was about 2.5 times that of the former. Moreover, the decay mode of Ba5(PO4)3OH was different from those of BaHPO4 and Ba3(PO4)2, which was ascribed to the effect of the substitution of three OH− for one PO43− on their electronic structures.

 Please wait while we load your content...
Please wait while we load your content...