Palladium-catalyzed oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide†
Abstract
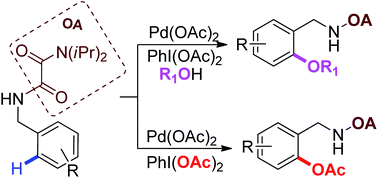
A practical palladium-catalyzed γ-oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide with PhI(OAc)2 as oxidant was developed. Selective alkoxylation or acetoxylation of oxalyl amide protected benzyl amine was achieved in high yield. The oxalyl amide protected α-substituted propylamines could be transformed into acetoxylated products in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...