Highly efficient removal of NO with ordered mesoporous manganese oxide at low temperature†
Abstract
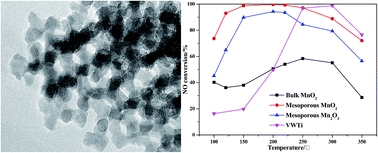
Highly ordered mesoporous MnO2 was prepared using KIT-6 as a hard template, for selective catalytic reduction (SCR) of NO with NH3 at low temperature, which was characterized using TEM, XRD, BET, XPS, H2-TPR, NH3-TPD and in situ DRIFT. Based on the results of HRTEM, the ordered mesoporous channels of MnO2 can be observed clearly. The SCR activity of NO with NH3 at low temperature was evaluated using these ordered mesoporous MnO2 as catalysts, and it was found that 100% NO conversion efficiency could be achieved at temperatures from 150 to 250 °C. For comparison, mesoporous Mn2O3 and bulk MnO2 were synthesized and their NO conversions were tested using the same parameters. The mechanism of improved SCR performance was investigated using XPS, H2-TPR, NH3-TPD and in situ DRIFT, and it was indicated that specific surface area, surface chemisorbed oxygen, reducibility and acid sites have great effect on the SCR reaction. In addition, the effects of H2O and GHSV on NO conversion were investigated.

 Please wait while we load your content...
Please wait while we load your content...