Novel and processable polyimides with a N-benzonitrile side chain: thermal, mechanical and gas separation properties
Abstract
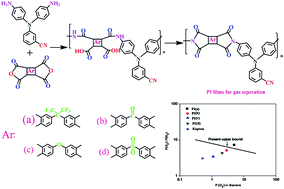
A series of organo-soluble and thermally stable polyimides (PIs) with a N-benzonitrile side group were synthesized from a new diamine monomer, 3-(bis(4-aminophenyl)amino)benzonitrile with four different aromatic dianhydrides via a thermal or chemical imidization method. The chemical structure and purity of the synthesized compounds were determined by different techniques. The inherent viscosities of the resulting PIs were in the range of 0.97–1.13 dL g−1 at a concentration of 0.5 g dL−1 in DMAc at 30 °C and these polymers were easily dissolved in organic solvents such as N-methyl-2-pyrrolidone, N,N-dimethylacetamide, and N,N-dimethylformamide, and tetrahydrofuran at room temperature. The obtained PIs showed a reasonably high glass-transition temperature (Tg up to 291 °C) and high thermal stability (T10 up to 420 °C). Flexible and transparent films were obtained easily by solution casting. The membranes of these polymers showed a tensile strength up to 115 MPa with elongation at break up to 13%, low water absorption (0.32–0.88%), low dielectric constant (1.98–2.38 at 1 MHz) and high optical transparency (λcut-off up to 352 nm). The combination of excellent solubility, film quality, and thermal stability makes these PIs potential candidates for high-performance gas separation membrane applications using a solution-casting processes. Permeability measurements were made for O2, H2, CO2 and CH4 at 35 °C.

 Please wait while we load your content...
Please wait while we load your content...