Co–salen complexes as catalysts for the asymmetric Henry reaction – reversed enantioselectivity through simple ligand modification†
Abstract
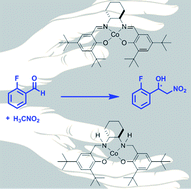
The asymmetric Henry reaction is an efficient method for the synthesis of enantioenriched nitro alcohols. We investigated the catalytic activity and enantioselectivity of derivatives of Co–salen [salen = N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexane-diamine] in this reaction. Tetrahydro-salen (salan) complexes displayed a reversed enantioselectivity compared to their Schiff-base counterparts. Oligomeric Co–salen catalysts showed a higher activity than their small molecule analogues, indicating a bimetallic pathway.

 Please wait while we load your content...
Please wait while we load your content...