Thermal oxidation reaction process and oxidation kinetics of abietic acid
Abstract
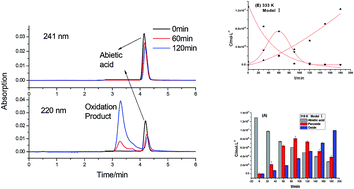
The thermal oxidation reaction process and oxidation kinetics of abietic acid were investigated by using self-designed gas–solid reaction equipment. The oxidation product and intermediates of the oxidation reaction of abietic acid were tracked by LC-MS. The results revealed a two-step oxidation reaction of abietic acid: abietic acid forms a peroxide first, followed by cracking to form hydroxyl-containing oxidized abietic acid. Both of the steps followed the pseudo-first order reaction kinetics, in which the kinetic equation of the first step is r1 = cA × 3.51 × 103 × exp(−58.96 × 103/RT), with activation energy of 58.96 kJ mol−1. The kinetic equation of the second step is r2 = cO × 6.09 × 105 × exp(−48.06 × 103/RT) with activation energy of 48.06 kJ mol−1. The kinetic equation of the total reaction is ra = ca × 1.12 × 106 × exp(49.51 × 103/RT) with apparent activation energy of 49.51 kJ mol−1.

 Please wait while we load your content...
Please wait while we load your content...