High performance solid state supercapacitor based on a 2-mercaptopyridine redox-mediated gel polymer
Abstract
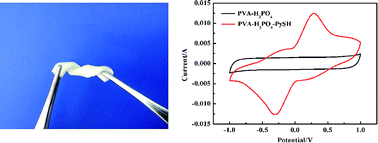
A novel gel polymer, polyvinyl alcohol-orthophosphoric acid-2-mercaptopyridine (PVA-H3PO4-PySH), is prepared through introducing redox-mediated 2-mercaptopyridine into a polyvinyl alcohol-orthophosphoric acid host, and a solid state supercapacitor is fabricated using the gel polymer as an electrolyte and a separator and using activated carbons as electrodes. The PVA-H3PO4-PySH gel polymer was shown to have excellent stretching and bending properties. The electrochemical properties of the supercapacitor are investigated by cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy. Surprisingly, electrode specific capacitance (1128 F g−1) and energy density (39.17 W h kg−1) are increased by 447% by introducing PySH as the redox mediator in the PVA-H3PO4 gel polymer. The supercapacitor with the PVA-H3PO4-PySH gel polymer shows an excellent capacitance retention of 80% for over 1000 cycles. Simultaneously, the ionic conductivity of the gel polymer electrolyte increased by 92% up to 22.57 mS cm−1 compared to that of the PVA-H3PO4 system. These improved performances are owed to the redox reaction between 2-mercaptopyridine (PySH) and 2,2′-bipyridine (PySSPy) redox couples in the PVA-H3PO4-PySH gel electrolyte, indicating the supercapacitor combines the double-layer characteristic of carbon-based supercapacitors and the faradaic reactions characteristic of batteries' energy-storage processes.

 Please wait while we load your content...
Please wait while we load your content...