Enhanced electrochemical performance of sulfur cathodes with a water-soluble binder†
Abstract
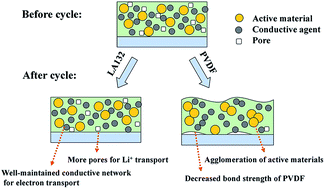
To improve the electrochemical performance of lithium–sulfur batteries, LA132, a kind of polyacrylonitrile is used as a water-soluble binder for sulfur cathodes. The optimal content of LA132 binder is investigated by electrochemical tests and morphology characteristics. Galvanostatic charge–discharge tests show that sulfur cathodes with 5 wt% LA132 (relative to the mass of whole cathode materials) exhibit a significant improvement in discharge capacity and cycle performance compared with the ones using 10 wt% polyvinylidene fluoride (PVDF) and LA132. Cyclic voltammetry and electrochemical impedance spectroscopy confirm that the LA132 sulfur cathodes show lower resistance and better kinetic characteristics. The effect of LA132 binder is further investigated via visual pictures and scanning electron microscopy (SEM). The visual picture shows the decreased bond strength of PVDF caused by its swelling, leading to a loss of active materials from the current collector. The morphologies of sulfur cathodes before and after 100 cycles indicate that the non-swellable LA132 could stabilize porous structures of cathodes during cycling and enhance the electrochemical performance of sulfur cathodes.

 Please wait while we load your content...
Please wait while we load your content...