Hierarchical CdS nanostructure by Lawesson's reagent and its enhanced photocatalytic hydrogen production†
Abstract
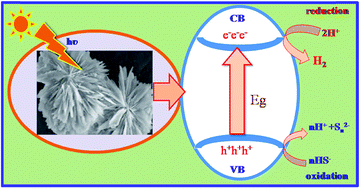
Lawesson's reagent (LR) has been effectively exploited for the synthesis of hierarchical architectures of cadmium sulphide (CdS) nanostructures for the first time. The X-ray diffractograms of the as synthesised CdS nanostructures confirm the formation of hexagonal CdS. The broadness of the XRD peaks clearly indicates the nanocrystalline nature of CdS with average crystallite size of 4 nm. A FESEM study revealed the formation of hierarchical nanostructures, whereas a TEM study showed that the hierarchical arrangement is composed of nanosized CdS particles. A band-gap i.e. 2.4 eV was derived from diffuse reflectance spectroscopy. The photoluminescence spectrum showed an emission peak at 535 and 568 nm which can be attributed to band-edge emission and surface emissions or possible metal vacancies, respectively. Considering the band-gap within the visible region, the photocatalytic hydrogen evolution performance of these CdS nanostructures was performed under visible light irradiation from hydrogen sulphide and water, respectively. Utmost hydrogen evolution i.e. 14 136 μmol h−1 g−1 and 2065 μmol h−1 g−1 was observed over a naked CdS nanostructure via H2S and water decomposition, respectively. The amount of hydrogen obtained by H2S splitting is much higher as compared to earlier reports.

 Please wait while we load your content...
Please wait while we load your content...