Synthesis and protein incorporation of azido-modified unnatural amino acids†
Abstract
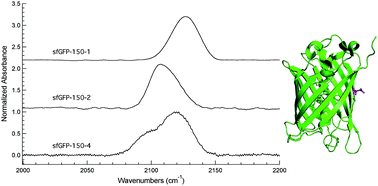
Two new azidophenylalanine residues (3 and 4) have been synthesized and, in combination with 4-azido-L-phenylalanine (1) and 4-azidomethyl-L-phenylalanine (2), form a series of unnatural amino acids (UAAs) containing the azide vibrational reporter at varying distances from the aromatic ring of phenylalanine. These UAAs were designed to probe protein hydration with high spatial resolution by utilizing the large extinction coefficient and environmental sensitivity of the azide asymmetric stretch vibration. The sensitivity of the azide reporters was investigated in solvents that mimic distinct local protein environments. Three of the four azido-modified phenylalanine residues were successfully genetically incorporated into a surface site in superfolder green fluorescent protein (sfGFP) utilizing an engineered, orthogonal aminoacyl-tRNA synthetase in response to an amber codon with high efficiency and fidelity. SDS-PAGE and ESI-Q-TOF mass analysis verified the site-specific incorporation of these UAAs. The observed azide asymmetric stretch in the linear IR spectra of these UAAs incorporated into sfGFP indicated that the azide groups were hydrated in the protein.

 Please wait while we load your content...
Please wait while we load your content...