Domino reaction of N-(cyanomethyl)-1,3-azolium quaternary salts with o-hydroxybenzaldehydes: scope and limitations†
Abstract
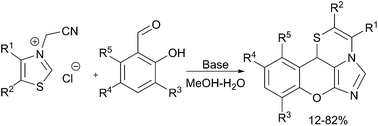
A route towards chromenes, annulated with an imidazo[5,1-c][1,4]thiazine core through a base-promoted domino reaction of thiazolium quaternary salts, has been developed. The synthesised compounds show high cytotoxic activity against human tumour cell lines.

 Please wait while we load your content...
Please wait while we load your content...