Multicomponent synthesis of pyridines via diamine functionalized mesoporous ZrO2 domino intramolecular tandem Michael type addition†
Abstract
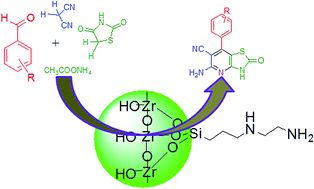
A new and straightforward synthetic method was developed for the facile synthesis of heterocycle-fused pyridine derivatives in aqueous media from Knoevenagel condensation between an aromatic aldehyde and an active methylene compound. This was followed by Michael type addition of a ketone to the activated double bond of the arylidene via intramolecular cyclization in the presence of diamine functionalized [N-(2 amino ethyl)-3-amino propyl trimethoxy silane (AAPTMS)] mesoporous ZrO2 (AAPTMS/m-ZrO2) to synthesize fused pyridines in high yield. This one-pot conversion, which involves multiple steps and requires no toxic/organic solvents, produced new C–C and C–heteroatom bonds with all reactants efficiently utilized.

 Please wait while we load your content...
Please wait while we load your content...