Palladium on manganese ferrite: an efficient catalyst for one pot synthesis of primary amides from iodobenzene†
Abstract
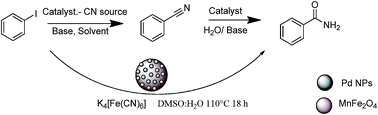
Amidation of aryl iodide in one pot is reported using Pd–MnFe2O4 as a catalyst. The catalyst was characterized by various techniques such as XRD, FEG-SEM, EDS, TEM, BET surface area and ICP AES. K4[Fe(CN)6]is used as a non toxic cyanation reagent for in situ generation of benzonitrile and hydrolyzed as soon as it formed. The catalyst was found to be efficient and can be used for several cycles without loss in activity. Good to excellent yields of primary amides were obtained.

 Please wait while we load your content...
Please wait while we load your content...