Lanthanide complexes with 3,4,5-triethoxybenzoic acid and 1,10-phenanthroline: synthesis, crystal structures, thermal decomposition mechanism and phase transformation kinetics†
Abstract
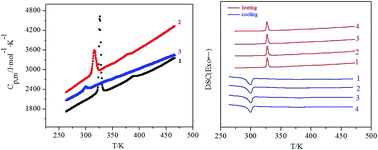
Three novel lanthanide complexes [Ln(3,4,5-TEOBA)3phen]2 (Ln = La(1), Pr(2), Eu(3); 3,4,5-TEOBA = 3,4,5-triethoxybenzoate; phen = 1,10-phenanthroline) were synthesized and characterized. Single crystal X-ray diffraction showed that the complexes are isostructural. Each complex has two center metals and each center is coordinated with seven oxygen atoms and two nitrogen atoms to form a distorted monocapped square antiprism geometry. A carboxylic group adopts three modes coordinated with center metal: bidentate chelate, bridging bidentate and bridging tridentate. The luminescence of complex 3 showed the characteristic emission of Eu3+ (5D0 → 7F0–3). The thermal decomposition mechanism of title complexes was studied by TG/DSC-FTIR technology. The heat capacities of complexes 1–3 were measured by DSC over the temperature range from 263.15 to 463.15 K. In the temperature range from 280 to 350 K, there was a solid-to-solid phase transition for each complex, which was further evidenced by four thermal circulating processes with the scanning rate of 10 K min−1. A study of the phase transition of four thermal circulating processes under different heating rates revealed a fine linear relationship between the activation energy (E) and the percent conversion (α). In the heating and cooling runs, supercooling was observed and the endothermic and exothermic enthalpies behaved differently.

 Please wait while we load your content...
Please wait while we load your content...