Structural characterization and functional evaluation of lactoferrin–polyphenol conjugates formed by free-radical graft copolymerization
Abstract
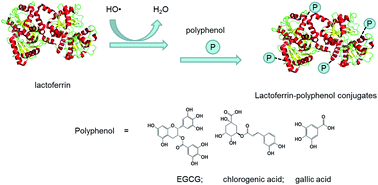
Covalent modifications of lactoferrin (LF) with epigallocatechin gallate (EGCG), chlorogenic acid (CA) and gallic acid (GA) were performed by adopting a free-radical grafting procedure in aqueous media. The resulting LF–polyphenol conjugates were characterized in terms of structural and functional properties. Results showed that the covalent binding amount into the LF molecule of EGCG, CA and GA was 68, 58 and 17 nmol mg−1, respectively. Covalent insertion of polyphenols into the LF molecule was verified by SDS-PAGE and MALDI-TOF-MS analysis, and in particular the molecular weight was increased from 84 011 Da (LF) to 85 906 Da (LF–CA conjugate). The circular dichroism and Fourier transform infrared spectroscopy analyses revealed that the content of α-helix increased and the contents of the remaining structures decreased, while the differential scanning calorimetry data indicated that the thermal stability of LF–polyphenol conjugates was enhanced after the modification. In addition, the antioxidant activity of LF–polyphenol conjugates was 0.23- to 2.10-fold (ABTS˙+ scavenging assay), and 0.04- to 2.19-fold (reducing power assay) higher than that of the control LF. Moreover, the covalent modification obviously changed the solubility and emulsifying properties of LF. The emulsifying properties of the LF–CA conjugate were better than those of the LF–EGCG and LF–GA conjugates.

 Please wait while we load your content...
Please wait while we load your content...