Can elongation of the π-system in triarylamine derived sensitizers with either benzothiadiazole and/or ortho-fluorophenyl moieties enrich their light harvesting efficiency? – a theoretical study†
Abstract
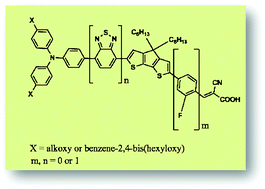
The structural and electronic properties of five known triarylamine derived sensitizers (A1, A1-F, C218, D2 and Y123) and their associated hypothetical dyes (C218-F, D2-F, Y123-F, Y1234 and Y1234-F) have been studied using density functional theory and time-dependent density functional theory. The sensitizers primarily comprise of a triphenylamine, a 4,4′-dihexylcyclopenta[2,1-b:3,4-b]dithiophene and a cyanoacrylic acid as the electron donating, π-spacer and accepting units, respectively. The π-system is extended by incorporation of either a benzo[c][1,2,5]thiadiazol-4,7-diyl unit or an ortho-fluorophenyl unit or both. To gain insight into the effect of elongation of the π-system on the electronic properties of dye sensitized TiO2 interfaces, first-principles calculations have been carried out on sensitizer molecules co-adsorbed on the (101) surface of the anatase TiO2. The theoretical results revealed that elongating the π-system of the sensitizers with both the benzothiadiazole and ortho-fluorophenyl units increases the molecular extinction coefficient, the excited state lifetime and the light harvesting efficiency but decreases the band gap and the reorganization energy relative to the structurally comparable reference dye Y123. The calculated short circuit current density and level alignment quality showed that the π-system in the triarylamine sensitizers elongated with both benzothiadiazole and ortho-fluorophenyl units broadens their potential use in DSSCs due to the enhanced values as compared to the reference dye. The results obtained in this study will provide a valuable reference for the strategy of inserting various π-spacers in triarylamine sensitizers for dye sensitized solar cell applications.
- This article is part of the themed collection: Organic chemistry collection

 Please wait while we load your content...
Please wait while we load your content...