Preparation and properties of magnetic Fe3O4/poly(pyrimidine-amide) nanocomposites: selective polyamidation of a bis(amino-pyrimidine-diol) compound in an ionic liquid†
Abstract
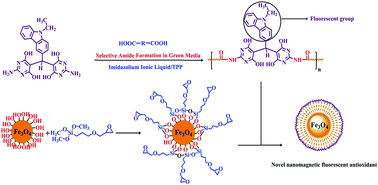
This work reports the preparation of magnetic nanocomposites (MNC)s from interfacial reaction between multifunctional poly(pyrimidine-amide)s (PPAs) and epoxide functionalized magnetic Fe3O4 nanoparticles. In this regard, organosoluble PPAs were synthesized selectively from polycondensation of bis(2-aminopyrimidine-4,6-diol) compound (DATPhP) with different aromatic and aliphatic dicarboxylic acids in a mixture of ionic liquid(IL)/triphenyl phosphite (TPP) at 110 °C for 2.5 h. The PPAs have weight-average molar mass (Mw) up to 49 500 g mol−1, glass transition temperatures (Tg) up to 240 °C and 10% weight loss temperatures (T10%) up to 486 °C (in N2). The results showed that strong chemical bonding between modified Fe3O4 nanoparticles and PPA chains reduced solubility and enhanced thermal and mechanical properties of the composites. Also, antibacterial and antioxidant activities of the monomer, PPAs and magnetic nanocomposite poly(pyrimidine-amide) (MNCPPA) were studied.

 Please wait while we load your content...
Please wait while we load your content...