Equilibrium and kinetic studies of Se(vi) removal by Mg–Al layered double hydroxide doped with Fe2+
Abstract
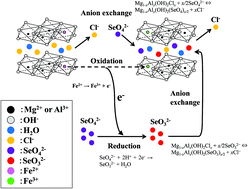
Mg–Al layered double hydroxide (Mg–Al LDH) doped with Fe2+ was found to be superior to undoped Mg–Al LDH in the removal of Se(VI) from aqueous solutions. For both systems, Se(VI) as SeO42− was removed through anion exchange with intercalated Cl−. In the Fe2+-doped Mg–Al LDH, however, some of the Se(VI) was reduced to Se(IV) upon oxidation of Fe2+ to Fe3+ in the LDH host layer to produce SeO32−, which was also adsorbed by the Fe2+-doped Mg–Al LDH through anion exchange. The reduction of Se(VI) to Se(IV) is advantageous for Se(VI) removal by Fe2+-doped Mg–Al LDH due to the larger charge density of SeO32−. The Fe2+-doped Mg–Al LDH effectively removed Se(VI) from an aqueous solution because of the anion exchange properties of Mg–Al LDH and activity of Fe2+ as a reducing agent. Se(VI) removal occurs through Langmuir-type adsorption, where the maximum adsorption and equilibrium adsorption constant were 1.4 mmol g−1 and 1.6, respectively. Se(VI) removal is well expressed as a pseudo second-order reaction. The apparent rate constants at 10, 30, and 60 °C were 1.2 × 10−3, 1.5 × 10−3, and 2.2 × 10−3 g mmol−1 min−1, respectively, and the apparent activation energy was 10.0 kJ mol−1. The rate-determining step is chemical adsorption through anion exchange of SeO42− and SeO32− with intercalated Cl−.

 Please wait while we load your content...
Please wait while we load your content...