Unusual electrochemical behaviour of AuBr4− in ionic liquids. Towards a simple recovery of gold(iii) after extraction into an ionic liquid
Abstract
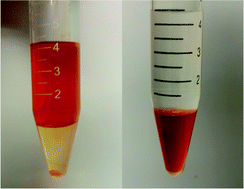
The electrochemistry of AuBr4− complexes extracted into ionic liquids [C8PYR][NTf2] or [C8MIM][NTf2] saturated with water and gas has been studied by cyclic voltammetry on a glassy carbon macro electrode and by linear voltammetry on a platinum microelectrode. Unlike AuCl4−, the reduction of AuBr4− to Au(0) is achieved following a one reduction step involving three electrons. The deposition of Au(0) from AuBr4− is carried out at a potential above that of water, leading to the simple and easy deposition of gold and subsequently to the extraction of AuBr4− into an ionic liquid.

 Please wait while we load your content...
Please wait while we load your content...