Quantum chemical insights into regioselective hydrolysis of C60F36†
Abstract
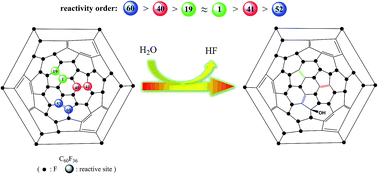
The hydrolysis reaction of fluorinated C60 to prepare fullerenols has been exposed by density functional theory for the first time. C60F36 was proved to hydrolyze via an SN2′ mechanism rather than SN1 or SN2 mechanism, and it is revealed that the hydrolysis is a regioselective reaction which may stem from the charge population of C60F36. The two adjacent carbon atoms connected by an isolated double bond possess opposite reactivities, and these two atoms exhibit a strong competition with each other to be attacked by water molecules during the hydrolysis. Bond order analyses using Wiberg indexes indicate that such a hydrolysis should be a concerted yet asynchronous reaction. Hydrolysis of fluorinated C60 can be considered as a method for fullerenol production via which structure-defined fullerenol will be obtained, and this method is greatly superior to traditional methods.

 Please wait while we load your content...
Please wait while we load your content...