Hydrothermal growth of large-size UO2 nanoparticles mediated by biomass and environmental implications
Abstract
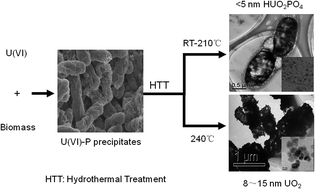
One difficult issue that environmental scientists are facing is how to convert soluble U(VI) into insoluble U(IV) and recycle it. In the present study, a method, which was widely reported in the literature, was used to collect soluble U(VI) using general biomass (including bacteria and yeast extract), and then a strategy was developed to transform the amorphous uranium-containing precipitates (Uranium–Phosphorus Amorphous Compound, UPAC) into large-sized insoluble UO2 nanoparticles. The results show that the biomass could precipitate more than 90% of the U(VI) (0.42 mmol L−1) within 10 min. The maximum precipitation capacity of the biomass (dry weight) ranged from 120 to 187 mg U g−1. The UPAC can be further converted into soluble uranyl phosphate compounds (HUO2PO4) at room temperature for 90 days or under the hydrothermal condition at 150 °C for 48 h. However, once the hydrothermal temperature was raised to 240 °C, insoluble UO2 nanoparticles of around 10 nm could be obtained within 48 h. This work provides a new possibility for the cost-effective preparation of nuclear fuel (UO2) with inexpensive raw materials. The mechanism correlating to the transformation of the UPAC into inorganic UO2 is also discussed here.

 Please wait while we load your content...
Please wait while we load your content...