Four d10 metal coordination polymers based on bis(2-methyl imidazole) spacers: syntheses, interpenetrating structures and photoluminescence properties†
Abstract
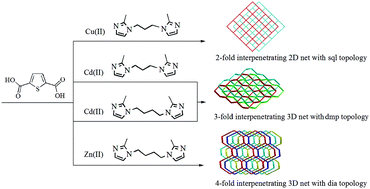
Two bis(2-methyl imidazole) ligands have been applied to assemble four interpenetrating coordination polymers with formula [Cu2Br2(bip)]n (1), [Cd(tdc)(bip)]n (2), [Cd(tdc)(bib)]n (3), and [Zn(tdc)(bib)]n (4) (tdc = 2,5-thiophenedicarboxylate anion, bip = 1,3-bis-(imidazol-2-methyl)propane and bib = 1,4-bis(imidazol-2-methyl)butane) under hydrothermal conditions. Structural analyses reveal that complex 1 exhibits a 2-fold interpenetrating 2D net with sql topology, whereas complexes 2 and 3 feature a 3-fold interpenetrating 3D net with dmp topology. Complex 4 displays a [2 + 2] 4-fold interpenetrating 3D net with dia topology. These results provide some insight into central metal ions, anionic ligands and bis(2-methyl imidazole) ligands' geometry-driven assembly of various interpenetrating CPs. Solid-state properties of these crystalline materials, such as thermal stabilities, and powder X-ray diffraction have been investigated. Moreover, the photoluminescence behavior of complexes 1–4 is also investigated.

 Please wait while we load your content...
Please wait while we load your content...