Conformational, vibrational and electronic structure investigations of (z)-2-(oxosilyl) ethylenol
Abstract
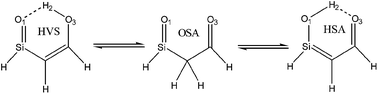
In the present work a detailed conformational analysis of (z)-2-(oxosilyl) ethylenol was performed using several computational methods, including MP2/6-311++G**, G2MP2 and B3LYP/6-311++G**. Harmonic vibrational frequencies were estimated at the same levels to confirm the nature of the stationary points found and also to account for the zero point vibrational energy (ZPVE) correction. In general, the chelated structures, HSA-1 and HVS-1, were more stable than the other conformers. This stability is mainly due to the formation of an O–H⋯O intramolecular hydrogen bond. The obtained results showed that the hydrogen bond strength is mainly governed by π-electron resonance inside the chelate ring. Hydrogen bond energies for HVS-1 and HSA-1 were obtained from the Shuster and Espinosa methods. The analysis of hydrogen bonds by quantum theory of atoms in molecules (AIM) and natural bond orbital (NBO) methods support the prior results. The electron density (ρ) and Laplacian (∇2ρ) properties, estimated by AIM calculations, indicate that the O⋯H bond possesses low ρ and positive (∇2ρ) values which are in agreement with the electrostatic character of the HBs, whereas the O–H bond has covalent character (∇2ρ < 0). The calculated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) with the frontier orbital gap are presented. The calculated HOMO and LUMO energies show that charge transfer occurs in the molecules. Further verification of the obtained transition state structures is achieved via intrinsic reaction coordinate analysis.

 Please wait while we load your content...
Please wait while we load your content...