A QM/MM study of the catalytic mechanism of α-1,4-glucan lyase from the red seaweed Gracilariopsis lemaneiformis†
Abstract
α-1,4-Glucan lyase (GLase, EC 4.2.2.13), a unique glycoside hydrolase family member, specifically cleaves the α-1,4-glucosidic linkages in glycogen, starch and malto-oligosaccharides to produce 1,5-anhydro-D-fructose from the non-reducing end. Previous studies have proved that GLase belongs to the retaining glycoside lyase, and the catalytic reaction contains both the glycosylation and deglycosylation/elimination steps, in which a covalent glycosyl–enzyme intermediate is involved. On the basis of the newly reported crystal structure of GLase (2X2I) and the speculated mechanism, the whole catalytic cycle of GLase has been studied by using a QM/MM method. Calculation results indicate that the whole catalytic cycle contains five elementary steps. Firstly, the aspartic acid residue D665 acts as an acid to protonate the glycoside oxygen, which is concerted with the cleavage of the glycoside bond. Then, the residue D553 functions as the nucleophile to attack the anomeric carbon to form the glycosyl–enzyme intermediate. Different from the retaining α-glucosidases whose glycosylation is a typical concerted process, the glycosylation process of glycosidic lyase follows a stepwise mechanism. For the deglycosylation/elimination step, two cases with or without the maltotriose group in the active site were considered. The departure of the maltotriose can facilitate the proceeding of this process. The deprotonated aspartic acid residue D553 further acts as a catalytic base to abstract the C2-proton of the glucosyl residue. The proton abstraction in the deglycosylation/elimination step is calculated to be the rate-limiting step of the whole catalytic reaction, which corresponds to the energy barriers of 20.69 and 18.53 kcal mol−1 for both of the two cases.
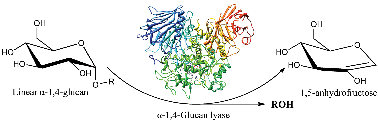

 Please wait while we load your content...
Please wait while we load your content...