Comparison of acidic site quantification methods for a series of nanoscopic aluminum hydroxide fluorides†
Abstract
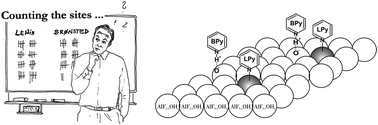
Quantitative determination of acidic surface sites is highly important for the characterization of solid acids because the activity of a catalyst is often related to the concentration of these sites. A recently developed method using 15N Nuclear Magnetic Resonance spectroscopy (NMR) for the quantification of acidic Lewis and Brønsted sites has been tested for a series of nanoscopic aluminum hydroxide fluorides. Comparison with other methods for the quantitative determination of acidic sites shows that this 15N NMR quantification method is a promising technique for the comprehensive investigation of acidic sites. Three different acidic sites, one Brønsted and two Lewis sites, can be distinguished by their 15N chemical shifts of pyridine and simultaneously quantified under conditions corresponding to catalytic reaction conditions. Determination of the individual concentrations of acidic sites allows further insight into the catalytic process. It was found that the concentration of Brønsted sites correlates with catalyzed conversion of citronellal to isopulegol in the investigated series of catalysts. Additionally, investigations indicate that one of the Lewis sites become blocked during the reaction of citronellal.

 Please wait while we load your content...
Please wait while we load your content...