Regio- and enantio-selective oxidation of diols by Candida parapsilosis ATCC 7330
Abstract
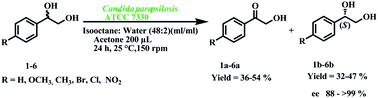
Selectivity between primary and secondary alcohols was observed in oxidation using whole cells of Candida parapsilosis ATCC 7330, where the secondary alcohol was preferentially oxidized. In racemic sec alcohols, the ‘R’ enantiomer was selectively oxidized to the corresponding keto alcohol (yield = 18–54%) leaving the ‘S’ diol (yield = 31–69% and enantiomeric excess from 14% to >99%). A biphasic system consisting of isooctane–water (48 : 2 v/v) was used as a medium for biotransformation at 25 °C. This is the first report of the regio- and enantio-selective oxidation of diols using C. parapsilosis ATCC 7330.

 Please wait while we load your content...
Please wait while we load your content...