Influence of anionic co-ligands on the structural diversity and catecholase activity of copper(ii) complexes with 2-methoxy-6-(8-iminoquinolinylmethyl)phenol†
Abstract
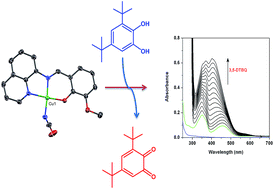
A novel dinuclear copper(II) complex, [Cu2L2(μ1,1-N3)2] (1), and a mononuclear copper(II) complex, [CuL(NCO)] (2), have been synthesized from a planar tridentate ligand 2-methoxy-6-(8-iminoquinolinylmethyl)phenol (HL) together with pseudohalides as coligands, and the solid state structures were determined by X-ray crystallography. Structural characterizations reveal that the geometry of centrosymmetrically related copper(II) centers in 1 is square pyramidal while it is square planar in 2. The impact of the structural diversity was found on their catechol oxidase mimicking activity. Strongly bridging azide ions being substitutionally inert mean complex 1 is inactive towards the catecholase activity, while mononuclear analogue 2 exhibits moderately strong catechol oxidase activity. The ESI-MS positive spectrum of a mixture of complex 2 and 3,5-DTBCH2 shows a peak corresponding to both superoxo and substrate bound species, Na[CuL(O2)(3,5-DTBCH)]+, suggesting that both the dioxygen and substrate simultaneously coordinated to the metal center in the catalytic cycle. Most importantly, complex 2 not only represents the mononuclear class of copper(II) compounds that are rarely visited for the study of catecholase mimicking activity but also the first example of a mononuclear square planar complex exhibiting catechol oxidase activity.

 Please wait while we load your content...
Please wait while we load your content...