Denitrification utilizing a vaporized enhanced-Fenton reagent: kinetics and feasibility
Abstract
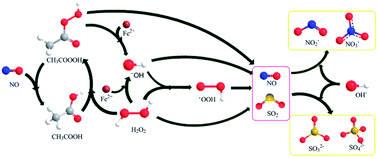
This paper proposes a novel integrative process for NO removal, in which NO was initially oxidized by a vaporized enhanced-Fenton reagent (EF), composed of hydrogen peroxide, ferrous and peroxyacetic acid (PAA), then absorbed by Ca(OH)2. The effects of EF constitution, the reaction temperature, the pH of EF solution and the SO2 concentration on NO removal were systematically investigated, and the experimental results indicated that both FeSO4 and PAA can significantly promote the oxidation rate of NO; the decreasing of pH and increasing of temperature played a key role in enhancing NO removal. The NO depletion exhibited a pseudo-first order kinetics pattern in 1–2 half-lives, based on the macrokinetics of NO oxidation. In addition, the rate constants determined in the temperature range of 60 to 120 °C were well fitted to the Arrhenius equation, yielding the apparent activation energy of 14.1 kJ mol−1. The mechanism of NO oxidation was also speculated.

 Please wait while we load your content...
Please wait while we load your content...