Synthesis of thiazolidines via regioselective addition of unsymmetric thioureas to maleic acid derivatives†
Abstract
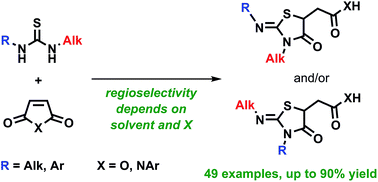
A wide range of unsymmetric thioureas has been studied in reaction with N-arylmaleimides and maleic anhydride. The regioselectivity of the addition depends not only on steric factors but on both solvent polarity and type of maleic acid derivative (imide or anhydride). The general regularities have been established providing practical guidelines to control the reaction result. The unequivocal structural assignment of all products has been done using NMR spectroscopy including 15N–1H HMBC experiments.

 Please wait while we load your content...
Please wait while we load your content...