Palladacycle-catalyzed Suzuki–Miyaura reaction of aryl/heteroaryl halides with MIDA boronates in EtOH/H2O or H2O†
Abstract
With good to excellent yields, a series of mono- or diheteroaryl compounds were synthesized via the palladacycle-catalyzed Suzuki–Miyaura reaction of various N-methyliminodiacetic acid (MIDA) boronates with aryl/heteroaryl halides in EtOH/H2O or H2O.
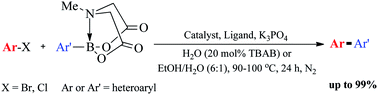

 Please wait while we load your content...
Please wait while we load your content...