Regio and stereoselective synthesis of β-keto functionalized C-glycosides via iron catalyzed Ferrier rearrangement reactions†
Abstract
An efficient iron-catalyzed decarboxylative C-glycosylation of glycals with β-keto acids via decarboxylative Ferrier rearrangement reaction has been established. This approach provides a wide range of β-keto-functionalized 2,3-unsaturated C-glycosides in moderate to good yields. Good selectivities are mainly observed with the use of D-galactal along with bulkier β-keto acids bearing phenyl moieties.
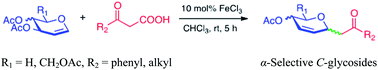

 Please wait while we load your content...
Please wait while we load your content...