Polyarylated boron-dipyrromethenes containing three different types of aryl groups†
Abstract
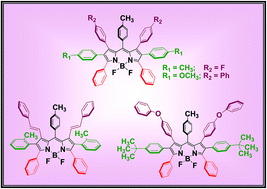
We report the synthesis and properties of the first examples of four novel multiply hexaarylated boron-dipyrromethenes (BODIPYs) containing three different types of aryl groups on each pyrrole ring of BODIPY. The BODIPYs were synthesized over a sequence of steps of bromination followed by Suzuki coupling with arylboronic acids. All reactions worked smoothly and we isolated the multiply polyarylated BODIPYs as stable fluorescent solids in high yields. The hexaarylated BODIPYs and associated reference compounds were confirmed by HR-MS and characterized by NMR, absorption, fluorescence and electrochemical techniques. The multiply polyarylated BODIPYs absorb strongly in the 550–600 nm region and emit in the 590–640 nm region with decent quantum yields and singlet state lifetimes. The polyarylated BODIPYs are very stable under redox conditions.

 Please wait while we load your content...
Please wait while we load your content...