Novel Burgess reagent mediated C-to-N aryl migration reaction in nitrones†
Abstract
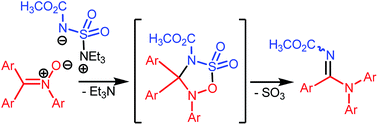
Nitrones undergo useful transformations with Burgess reagent. The reaction ostensibly involves a [3 + 2] annulation across a σ-bond followed by rearrangement involving C-to-N aryl migration. On the basis of available experimental evidence, plausible mechanisms for the rearrangement and the overall conversion have been proposed.

 Please wait while we load your content...
Please wait while we load your content...