Phase separation in aqueous systems for realizing virtually significant extractions†
Abstract
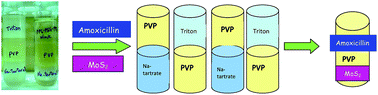
Polyvinylpyrrolidone (PVP) forms aqueous biphasic systems with tri-block co-polymers, surfactants and salts. The phase diagrams of PVP–salt and PVP–surfactant systems were constructed by a turbidometric titration method at 296 K. Density measurements of the phase forming solutions at this temperature also led to an attractive outcome of the formation of two tri-phasic systems among the components of our study (Na-tartrate + PVP + Triton-X-100 and Na-tartrate + PVP + PPG–PEG–PPG). We also constructed a spreadsheet reflecting the relationship between miscibility and the possibility of phase separation between the components, consisting of the seven phase forming solutions to open up different possibilities of ABS formation. Some completely new biphasic systems were obtained as well as the process of gel formation of a PVA solution in the presence of salt and polymer solutions was also observed. The applications of some of the developed ABSs are described for the recovery of an antibiotic drug, amoxicillin and a catalyst, molybdenum disulphide.

 Please wait while we load your content...
Please wait while we load your content...