Self-radiolysis of tritiated water. 3. The ˙OH scavenging effect of bromide ions on the yield of H2O2 in the radiolysis of water by 60Co γ-rays and tritium β-particles at room temperature
Abstract
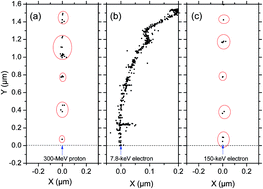
Monte Carlo track chemistry simulations were used to determine the yields (or G-values) of hydrogen peroxide in the radiolysis of neutral water and dilute aqueous bromide solutions by low linear energy transfer (LET ∼ 0.3 keV μm−1) radiation (e.g., γ-rays from 60Co, fast electrons or high-energy protons) and tritium β-particles (mean LET ∼ 6 keV μm−1) at 25 °C. We investigated the influence of Br− ions, as selective scavengers of ˙OH radical precursors of H2O2, on the inhibition of G(H2O2) for these two types of radiation. Studying this system under a wide range of Br− concentrations (5 × 10−7 to 0.2 M) and using a well-accepted mechanism for radiolysis in the presence or absence of air, we examined the chemical changes in the scavengeability of H2O2 produced by 300 MeV irradiating protons (used in this work to reproduce the effects of 60Co γ/fast electron radiolysis) and tritium β-electron radiolysis. We found that these changes could be related to differences in the initial spatial distributions of radiolytic species (i.e., the structure of the electron tracks, the low-energy β-electrons of tritium depositing their energy almost entirely as cylindrical “short tracks” and the energetic Compton electrons produced by γ-radiolysis forming mainly spherical “spurs”), in full agreement with previous experimental and theoretical work. Simulations showed that the short track geometry of higher LET tritium β-electrons in both water and aqueous bromide solutions favored a clear increase in G(H2O2) compared to 60Co γ-rays. Moreover, the presence of oxygen was seen to scavenge hydrated electrons (eaq−) and H˙ atoms on the ∼10−7 s time scale, thereby protecting H2O2 from further reactions with these species in the homogeneous stage of radiolysis. This protection against eaq− and H˙ atoms therefore led to an increase in the long time H2O2 yields, as seen experimentally. Finally, for both deaerated and aerated solutions, the H2O2 yield in tritium β-radiolysis was found to be more easily suppressed than in the case of cobalt γ-radiolysis, and interpreted by the quantitatively different chemistry between spurs and short tracks. These differences in the scavengeability of H2O2 precursors in passing from 300 MeV irradiating protons to tritium β-electron irradiation were in good agreement with experimental data, thereby lending strong support to the picture of tritium β-radiolysis in terms of short tracks of high local LET.

 Please wait while we load your content...
Please wait while we load your content...