Steric effect on the self-assembly behaviours of amino acid derivatives†
Abstract
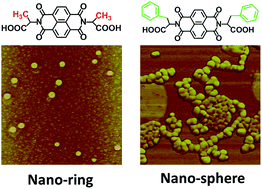
To investigate the steric effect of amino acids, N,N′-dialanine-1,4,5,8-naphthalene tetracarboxylic acid diethylamide (ANTA) and N,N′-diphenylalanine-1,4,5,8-naphthalene tetracarboxylic acid diethylamide (PNTA) were synthesized based on naphthalene-1,4,5,8-tetracarboxylic dianhydride (NTA) with alanine and phenylalanine, respectively. These compounds were characterized by infrared spectroscopy (IR), magnetic resonance spectroscopy (1H NMR, 13C NMR) and mass spectrometry (MS). Interestingly, ANTA could self-assemble into spiral nanorings, but PNTA formed nanospheres. In order to reveal the self-assembly driving forces, the two-dimensional (2D) supramolecular self-assembly behaviours were studied on highly oriented pyrolytic graphite (HOPG) by scanning tunneling microscopy (STM) at the solid–liquid interface, which indicated the driving forces could be attributed to intermolecular hydrogen-bonding and π–π stacking interactions. These results represent different self-assembly behaviours due to the steric effect of amino acids.

 Please wait while we load your content...
Please wait while we load your content...