Simultaneous adsorption of nitrogenous heterocyclic compounds by granular activated carbon: parameter optimization and multicomponent isotherm modeling
Abstract
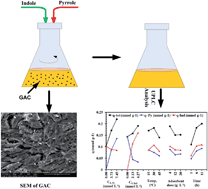
Many industrial aqueous streams contain nitrogenous aromatic compounds, which need to be removed by an adsorption process, for which it is essential to study the interactive affects of these compounds during their simultaneous adsorption. In the present study, granular activated carbon (GAC) was used for simultaneous adsorption of pyrrole and indole from aqueous solution. First, Taguchi's method (L27 orthogonal array) was applied to optimize various parameters, like initial adsorbate concentration, adsorbent dose, temperature and contact time for their simultaneous adsorption onto GAC. Adsorbent dose and the interaction between initial concentrations were found to be the most significant factors. Indole adsorption onto GAC was found to be higher than that of pyrrole. Thereafter, binary adsorption equilibrium data were generated and modeled by various multicomponent isotherm models. Extended-Langmuir isotherm best represented the isotherm data at 30 °C among various multicomponent isotherm models used in this study.

 Please wait while we load your content...
Please wait while we load your content...