Size-dependent thermal decomposition and kinetics of ultrafine alkali metal styphnates
Abstract
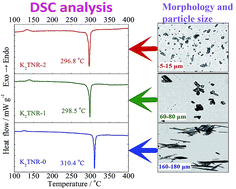
Three alkali metal styphnates, K2TNR, Rb2TNR and Cs2TNR, were fabricated into different particle sizes by the crystal-morphology-control microemulsion synthesis. The thermal decomposition and kinetics were studied by DSC and DPTA techniques. As the particle size decreases, the thermodynamic and kinetic parameters decrease while the decomposition gas amounts and reaction rate constants increase. The reduction in particle size leads to an increase in reactivity and a decrease in thermal stability. The thermal sensitivity, determined by 5 s explosion temperature and flame sensitivity, grows with decreasing particle size. The ultrafine materials have small particle size and large specific surface area, and therefore possess strong surface bonding energy, low activation energy barrier and high reactivity. In the case of the same size, the order of thermal stability is Cs2TNR < Rb2TNR < K2TNR. The central metal of small atomic radius has strong coordination bonding energy and therefore determines the high stability.

 Please wait while we load your content...
Please wait while we load your content...