Choline chloride based eutectic solvents: direct C-3 alkenylation/alkylation of indoles with 1,3-dicarbonyl compounds†
Abstract
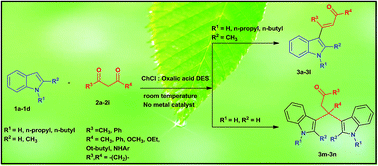
C-3 alkenylation/alkylation of indoles depends on the position of the substituent on the indole used in the reaction. C-2 substituted indole results in the formation of C-3 alkenylated indole derivatives, whereas plain indole gives rise to the bis(indolyl)carbonyl derivative instead of the C-3 alkenylation product under the same set of reaction conditions. Deep eutectic mixtures are cost-effective, and bio-degradable.

 Please wait while we load your content...
Please wait while we load your content...