Catalytic hydrogen evolution from hydrolytic oxidation of organosilanes with silver nitrate catalyst†
Abstract
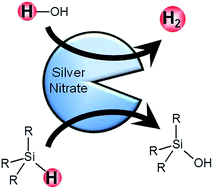
In the light of uncertainty over the amount of recoverable fossil fuel reserves, hydrogen is touted to be a promising energy carrier in the future. Nevertheless, hydrogen storage remains a daunting challenge but a potential reaction for the generation of hydrogen on demand is the hydrolytic oxidation of organosilanes. Here, we demonstrate that silver nitrate, a readily available ionic salt, can catalyze the hydrolysis of organosilanes to produce hydrogen and organosilanols. In particular, turnover numbers and turnover frequencies in excess of 5 × 103 and 102 min−1 respectively are obtainable for the hydrolysis of triethylsilane at room temperature. This proposed silver nitrate mediated system is, by far, the simplest and cheapest catalytic hydrolysis of organosilanes. Results from the kinetic studies suggested a mechanistic scenario in which the hydrolysis of organosilanes is third order overall and first order in organosilane, water, and catalyst. The high hydrogen yield observed makes the silver nitrate catalyst an attractive material for hydrogen evolution.

 Please wait while we load your content...
Please wait while we load your content...