Exploiting Na2MnPO4F as a high-capacity and well-reversible cathode material for Na-ion batteries†
Abstract
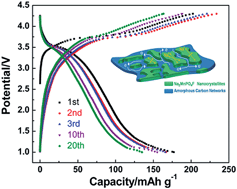
A Na2MnPO4F/C nanocomposite material is successfully synthesized via spray drying, followed by a high-temperature sintering method. It is shown that the highly phase-pure Na2MnPO4F with symmetry of the P21/n space group is uniformly embedded in the carbon networks, which play a key role in building up a highly efficient, electron-flow channel and elevating the electronic conductivity of the nanocomposites. The electrochemical measurements show that the initial discharge capacity of Na2MnPO4F reaches up to 140 and 178 mA h g−1 at 30 °C and 55 °C, respectively. Furthermore, the capacity still maintains 135 mA h g−1 after 20 cycles at 55 °C. The Na+ diffusion coefficient in Na2MnPO4F is calculated at about 10−17 cm2 s−1 by the GITT method. The impressive cycling performance of the material is ascribed to the good structural reversibility and stability of Na2MnPO4F, which are confirmed by the ex situ XRD measurements during the first cycle and after 30 cycles.

 Please wait while we load your content...
Please wait while we load your content...