Divergent reaction: metal & oxidant free direct C–H aryloxylation and hydride free formal reductive N-benzylation of N-heterocycles†
Abstract
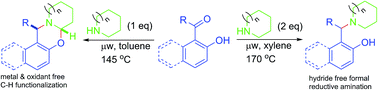
Metal, oxidant and other additive-free novel methods for direct C–H aryloxylation of aliphatic amines are developed. In the presence of excess amine, the course of the reaction was diverted, producing various arylmethylamines via hydride-free formal reductive amination. Involvement of a quinone methide intermediate was revealed from mechanistic studies.
- This article is part of the themed collection: How does it work? – a collection on mechanistic studies

 Please wait while we load your content...
Please wait while we load your content...