An efficient Fe2O3/HY catalyst for Friedel–Crafts acylation of m-xylene with benzoyl chloride
Abstract
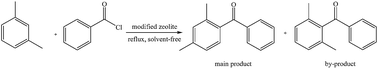
Iron oxide supported on HY zeolite was prepared and exhibited excellent catalytic performance in the acylation of m-xylene with benzoyl chloride. It was characterized by XRD, BET, XPS, NH3-TPD and Py-IR. The obtained results indicated that the catalytic activity of Fe2O3/HY is enhanced with the increase of Lewis acidic sites. Furthermore, the reaction parameters, including load of Fe2O3, temperature, molar ratio and the dose of catalyst, were optimized. Thus the acylation proceeds effectively to afford 2,4-dimethylphenylacetophenone in 94.1% yield under optimum conditions. Finally, the catalyst was examined for the acylations of a series of arenes, all of the alkyl substituted benzenes were transformed to the corresponding products in satisfactory yields while the acylation of chlorobenzene was sluggish. The catalyst was easily separated from the reaction mixture and reused for five runs without appreciable loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...