Effect of pH on the liquid superlubricity between Si3N4 and glass achieved with phosphoric acid
Abstract
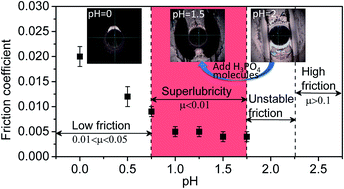
In the present study, the pH dependence of liquid superlubricity between Si3N4 and glass achieved with phosphoric acid solution was investigated. It is seen that the superlubricity can be achieved only when the pH value is in the range of 0.75–2. To reveal the mechanism, the evolutions of confined solutions with different pH values between two friction surfaces were investigated by an online observation. It was seen that the superlubricity appeared when the confined solution between the two friction surfaces forms a starvation state. When the pH is in the range of 0.75–1.75, the starvation state can be formed as long as the running-in period is end. When the pH is in the range of 1.75–2, the starvation state can be formed by adding a certain amount of phosphoric acid molecules in the contact region, which leads to the transformation of an unstable friction state to a superlubricity state. When the pH is in the range of 0–0.75, the superlubricity cannot be obtained, no matter how the test conditions are changed because of the high contact pressure and lack of time for the tribochemical reaction to take place between the friction surfaces and hydrogen ions. When pH is greater than 2, the value of friction stays high because the amount of hydrogen ions adsorbed on the friction surfaces are not sufficient to make the surfaces positively charged.

 Please wait while we load your content...
Please wait while we load your content...