Superacid BF3–H2O promoted benzylation of arenes with benzyl alcohols and acetates initiated by trace water†
Abstract
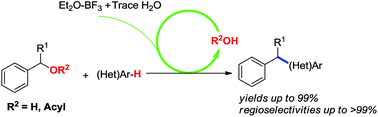
A convenient procedure employing simple starting materials benzyl alcohols and acetates as the benzyl donors to assemble a series of diarylalkanes through benzylation of arenes using in situ prepared superacid BF3–H2O as an efficient promoter has been developed. The beneficial role of water in the reaction has been clarified with combination of control experiments and 11B NMR analysis. This reaction is a self-promoted model, which is triggered by the trace of water and continuously promoted by self released by-product water (or carboxylic acid). A wide range of substrates are investigated and the moderate to excellent yields and the good regioselectivities for secondary benzyl alcohols as well as arenes bearing electron-withdrawing groups have been achieved. As a result, moisture in the reaction system has been utilized as an efficient initiator in all benzylation cases.

 Please wait while we load your content...
Please wait while we load your content...