Synthesis of amorphous porous zirconium phosphonate materials: tuneable from micropore to mesopore sizes†
Abstract
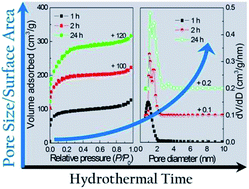
Amorphous porous zirconium phosphonate materials constructed from 1-hydroxyethylidene-1,1′-diphosphonic acid, having tunable sizes from micropore to mesopore, were hydrothermally synthesized in a CTAB–H2O–ethanol ternary system (CTAB = cetyltrimethyl ammonium bromide). The as-synthesized materials were mesostructured, and could be transformed into (super-)microporous hybrid solids after a surfactant-extraction process. By varying the hydrothermal time, the pore sizes of the obtained zirconium phosphonates could be efficiently tuned from the micropore (0.87 nm) to mesopore (2.5 nm) range, and their micropore specific surface areas ranged from 116 to 509 m2 g−1 with pore volumes in the range of 0.11–0.35 cm3 g−1. To rationalize the formation of microporosity from mesostructure, a working hypothesis of 2-step condensation of Zr–OH groups with RP–OH species involving one step in the mesostructured phase formation period and the other in the surfactant removal process is proposed according to the XRD, TEM, FT-IR and XPS analyses of the samples before and after surfactant removal. Considering the acidity and tunable pore sizes, the prepared porous zirconium phosphonates may find their potential applications in adsorption, shape-selective heterogeneous catalysis, ion exchange and proton conduction.
- This article is part of the themed collection: Porous Materials (FEZA 2014)

 Please wait while we load your content...
Please wait while we load your content...