Ferroelectric-like structural transition in metallic LiOsO3
Abstract
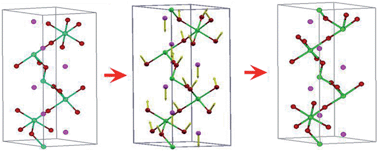
It is assumed that ferroelectric behaviour only appears in the materials with an energy gap. However, a ferroelectric-like structural transition has been experimentally explored in metallic LiOsO3 [Nature Materials 12, 1024 (2013)]. In this paper, we investigate the possible origin of a ferroelectric-like transition and its effect on the electronic structure through theoretical simulations. By performing first-principles calculations, we found that the topologically compact structures formed by strong Os–O bonds forces Li ions to be displaced, which is responsible for the ferroelectric-like structural transition. Our calculations also indicate that the chemical valence should be Lin+[OsO3]n−, where n < 1. Furthermore, the intrinsic electric dipoles induced by the ion displacement are spontaneously screened by the remanent electrons of Li ions. Thus, the explored mechanism of the ferroelectric-like transition in metallic LiOsO3 may help develop other ferroelectric materials.

 Please wait while we load your content...
Please wait while we load your content...