Thermal oxidation to regenerate sulfone poisoned Pd-based catalyst: effect of the valence of sulfur
Abstract
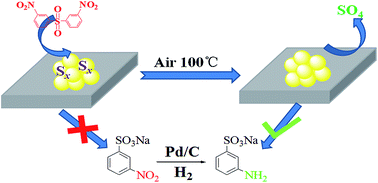
Sulfur deactivation is a serious problem which largely limits the industrial application of noble metals as catalysts. Here we report a thermal oxidation method to regenerate sulfone poisoned Pd/C catalyst applied in the hydrogenation of sodium-m-nitrobenzene sulfonate (SNS). It was found that the initial activity of Pd/C catalyst could be substantially recovered after treating it in air at temperatures as low as 100 °C. And the catalyst could be reused for at least 20 times without the significant loss of activity. The properties of deactivated and regenerated catalysts were studied in detail by BET measurement, X-ray photoelectron spectroscopy (XPS), temperature programmed desorption (TPD), and Fourier transform infrared spectroscopy (FT-IR). The results indicated that the main surface sulfur species found on deactivated and regenerated Pd surfaces were Sn and sulfate (SO4), respectively. The change of the valence of sulfur species was found to be the key factor influencing the catalytic activity of the Pd-based catalyst.

 Please wait while we load your content...
Please wait while we load your content...